Josella Hunt
~
Biosciences Department, Wallace Building, Swansea University, SA2 8PP
Abstract
Migration is an intrinsic aspect of the life history of many baleen whale species. It is assumed that they occupy high latitudes during the summer months to exploit prey aggregations created by high productivity, and then fast during a migration to low latitudes in the winter to mate and give birth. However, this migratory paradigm has been developed from a few well-known species. Therefore it may not be accurate to use to explain the migrations of lesser known species, such as the blue whale, Balaenoptera musculus; especially as inter- and intraspecific diveristy is very common with the cetacean group. Resultantly, predictions on how anthropogenic threats, such as climate change, will affect these species could be inaccurate, and lead to ineffective conservation. This paper reviews the distributions and migrations of blue whale populations worldwide, the potential effects of climate change on their migration and distribution, and how conservation could be affected in the future. Contrary to the paradigm, blue whales do not migrate exclusively for breeding. They track seasonal resources, feeding throughout the year; with mating and calving appearing opportunistic, or of secondary importance at low-latitude sites. Populations such as Australian pygmy, North Atlantic and Northeast Pacific blue whales have been relatively well-studied using a variety of methods; however the majority of other populations need much more research. Their reliance on primary productivity hotspots makes them vulnerable to climate change. The localities and quantities of their prey are expected to alter worldwide, meaning blue whales may aggregate in different areas or have to travel larger distances for lower quality resources. Consequently, protected areas and monitoring programmes could be ineffective if whales no longer utilise the regions they cover. Negative climate change-associated population effects, coupled with a higher frequency of anthropogenic impacts due to ineffective conservation could cause localised extinctions for some populations already at low levels. It may be possible to detect future climate-induced changes in distribution and plan conservation accordingly; however research needs to first become a lot more extensive, so that deviations can be detected.
© gentlegiants.is
I. Introduction
(1) The migratory paradigm of baleen whales
Migration is the recurrent, regular movement of individuals between different parts of a home range, and is a key part of the life history of many baleen whale species (Dingle, 1996; Clapham, 2001). The generally accepted paradigm is that high latitude areas are utilised for feeding during the austral or boreal summer, when seasonal productivity in these areas are at their highest (Corkeron & Connor, 1999; Robbins et al., 2011). Populations then travel to low latitude waters during the austral or boreal winter, fasting during this portion of the year, to calve and mate (Clapham, 2001).
However, overall very little is known about baleen whale movement. Past exploitation by whaling, their high mobility, and the amount of time which they spend beneath the surface makes elucidating their migratory destinations and pathways difficult (Bose & Lien, 1989; Roman & Palumbi, 2003). The above paradigm has been formed from data collected on very well studied species, such as the humpback whale, Megaptera noveangliae (Clapham, 2001), but despite this it is often extrapolated to lesser known species. This may be inaccurate, as within the cetacean group foraging strategies, movement patterns, and dialects are highly diverse, including at an intraspecific level (Pitman et al., 2011). Therefore, even for species taxonomically close to the humpback whale, their patterns of, and motivations for migration could be very different.
(2) Climate change, baleen whale migration, and conservation
Migratory species worldwide are subject to many anthropogenic threats, such as obstacles, barriers, habitat destruction, hunting and climate change (Wilcove & Wilkelski, 2008). It may first appear that baleen whales are immune to many of the threats which other (mainly terrestrial) species face; living in a liquid medium with very few boundaries means that barriers and obstacles are unlikely to impede their movement, and full habitat destruction often does not occur (Simmonds & Isaac, 2007). However, evidence has already shown that baleen whales are at risk from anthropogenic climate change. The last century has experienced the biggest increase in global temperature than any other in the past 1000 years; which has been attributed to deforestation and the burning of fossil fuels, causing climatic and ecological changes in the world’s oceans (Osborn & Briffa, 2006). The migratory timing of humpback whales is altering (Ramp et al., 2015), and a mass stranding of hundreds of gray whales, Eschrichtius robustus was attributed to climate related decreases in their amphipod prey further north, causing starvation during their southward migration (Moore et al., 2003).
Climate change is also likely to affect the migratory routes of baleen whales, which presents a significant problem for conservation attempts (Simmonds & Elliot, 2009). Current monitoring efforts and research programs are based in areas where known aggregations occur at some point of the year, such as Hawaii and the Californian coast (Mate, 1999). The few marine protected areas which have been created for baleen whales are also based on known distributions, such as right whale, Eubalaena glacialis, critical habitat off Massachusetts and Georgia (Ward-Geiger et al., 2005). If these aggregations and the migratory pathways between them were to change, then endangered populations could be subject to a higher frequency of anthropogenic impacts, leading to detrimental population effects (Randage et al., 2014). Consequently it is important to establish the known distribution of under-researched species, identify data gaps, and use the knowledge of their migration to predict how they might respond to future climate change, and how this would impact proposed conservation measures.
(3) Topic and aims of the review
The blue whale is the largest species of cetacean, reaching lengths of up to 30 m (figure 1; Sears & Perrin, 2011). It was subject to mass overexploitation by commercial whaling; some populations have been reduced to <1 % of their original levels and a few appear to be locally extinct (Branch et al., 2007). Resultantly there is a paucity of information on its current distribution and movement patterns, even so the migratory paradigm is presently assumed to apply to this species.
Figure 1. Blue whale, Balaenoptera musculus, size in comparison to a human.
This paper will review what is currently known on the migratory patterns of different blue whale populations worldwide, to investigate whether the migratory paradigm does apply and identify any data gaps which need to be filled. The collated information will then be used to assess how climate change may impact this species, and what knock-on effects this may have for its conservation.
II. Blue whale migratory patterns
(1) Blue whales worldwide – subspecies and distinct populations
As a whole blue whales have an almost worldwide distribution (figure 2), although distinct subpopulations and subspecies inhabit specific oceanic regions (Branch et al., 2007). In the North Atlantic and Pacific, a single species, Balaenoptera musculus, is recognised (Burtenshaw et al., 2004; Pike et al., 2009). However, the Southern Hemisphere contains two subspecies; the Antarctic blue whale (Balaenoptera musculus intermedia), and the pygmy blue whale (Balaenoptera musculus brevicauda; Ichihara, 1966; Branch et al., 2007). In comparison to Antarctic blue whales, pygmies are shorter both at sexual maturity and their maximum length, 19.2 m vs. 23.7 m and 24.1 m vs. >30 m respectively (Macintosh et al., 1929; Ichihara, 1966). They also differ in other physical features, call repertoires (which is how they are sectioned in this review) and genetics (Conway, 2005; Stafford et al., 2004). A range of methods can be used to investigate baleen whale migration, all of which have their advantages and limitations (table 1). Therefore in the subsequent sections, studies of various methods will be used to review the distribution and movement patterns of each population, as this is likely to result in more accurate conclusions.
Figure 2. The summarised distribution of blue whales (bright blue) as a whole worldwide. © WDC.org.uk.
(2) The Southern Hemisphere
(a) Antarctic blue whales (B. m. intermedia)
Antarctic blue whales are found throughout the Southern Ocean (figure 3; Branch et al., 2007), although their range has shifted polewards since the cessation of whaling. Migration in this subspecies is dynamic, with some individuals migrating to lower latitudes during the winter, and some remaining in polar waters year round (Širović et al., 2004; Stafford et al., 2004; Širović et al., 2009; Thomisch et al., 2016). Passive acoustic monitoring (PAM) hydrophones deployed in the Weddell Sea recorded vocalisations most frequently during the summer (reflecting the known seasonal feeding aggregations which occur here), however they were still recorded during the winter at latitudes of 69° S in regions almost completely covered in sea ice (figure 3; Thomisch et al., 2016). It is still unknown why a proportion of the population remains at high latitudes during the winter. If this subspecies does migrate for breeding then it could be that non-breeding individuals do not migrate; conversely if resources are a migratory driver then there could be sufficient krill to support a subset of resident or non-migratory whales.
|
Data Type |
Method of Collection |
Advantages |
Disadvantages |
References |
|
Line-transect sampling |
Sightings recorded from a boat or plane travelling along designated lines |
– Even sampling
– Relatively easy to design and implement |
– Bad weather and logistics can cause uneven sampling
– Some animals excluded by perception or availability bias |
Buckland et al., 2001 Redfern et al., 2006 Marsh & Sinclair, 1989 |
|
Passive Acoustic Monitoring |
Bottom mounted benthic or floating unmanned hydrophones |
– Cheaper than line-transects
– Operate in all weather conditions – Detect large spatial and temporal scale movement – Data can be sourced from Navy hydrophones |
– Not possible to quantitatively estimate abundance
– Long data processing times subject to bias |
Redfern et al., 2006 Di Sciara & Gordon, 1997 Samaran et al., 2013 |
|
Tagging devices |
Satellite telemetry tags attached to individuals |
– Highly accurate movement data
– Can also give behavioural information |
– Expensive
– Small sample numbers – Behavioural effects – Anomalies frequent |
Redfern et al., 2006 Burtenshaw, 2004 Double et al., 2014 |
|
Mark-recapture |
Identified individuals photographed in different locations |
– Inexpensive
– Can use public data – Tracks individuals – Can give long term data |
– Identification inaccuracies
– Photos have to be high quality and right perspective – Data processing can be long |
Redfern et al., 2006 |
|
Historic whaling records |
Location data from whaling used to give information on distribution |
– Infer movement patterns when populations were larger
– Can give information on seasonal distribution |
– Subject to effort bias – were individuals absent from an area or was it not whaled? |
Gregr, 1992 Redfern et al., 2006 |
|
Strandings |
Location data from strandings used to give information on distribution |
– Large strandings databases often available
– Inexpensive method of data collection |
– Cannot tell where the individual died
– Often only reported in populous areas |
Rice, 1998 |
|
Genetics |
Comparing DNA of blue whale aggregations |
– Good for establishing population connectivity
– Can estimate when populations split |
– Often small sample sizes
-Invasive collection methods – Need to have microsatellite markers to extract DNA |
Torrez-Florez et al., 2012 |
Table 1. Advantages and disadvantages of methods used to infer blue whale movement and migratory patterns.
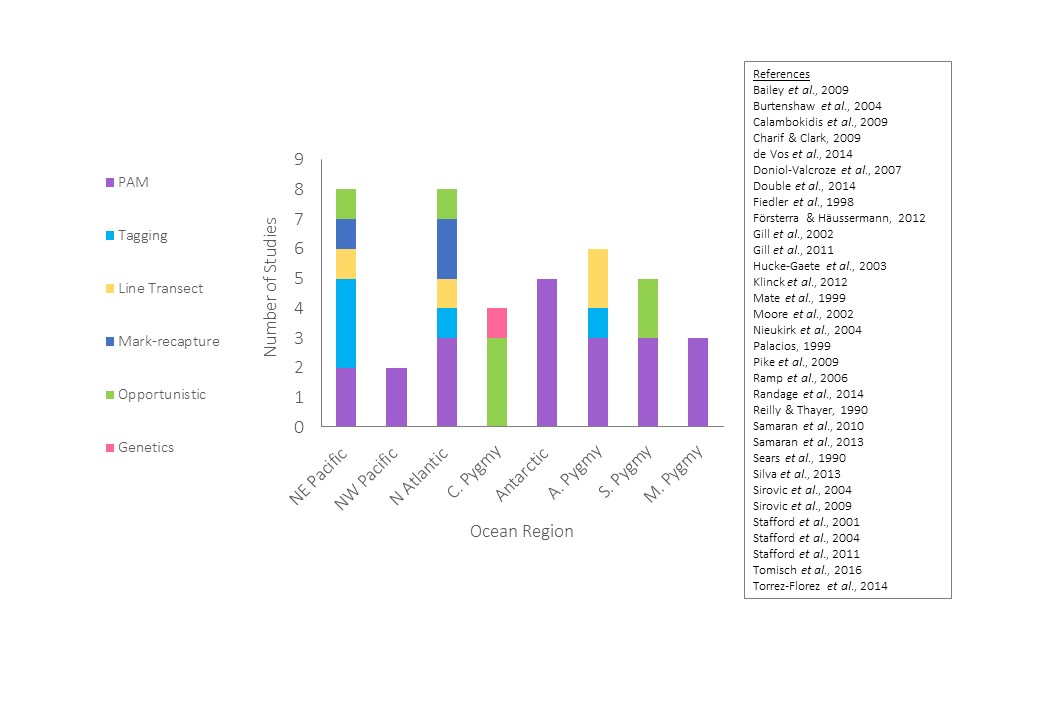
During the austral winter animals have been recorded in many lower latitude regions across the Southern Hemisphere, such as southwest Australia, and even as far north at the Eastern Tropical Pacific (ETP; figure 3; McCauley et al., 2004; Stafford et al., 2004; Samaran et al., 2013). One potential migratory cycle has been identified by PAM hydrophones off Madagascar and Île Amsterdam, further south; the more southerly hydrophones recorded more calls overall, and on the northern one, vocalisations where much more frequent during the winter than the summer (Samaran et al., 2013). However this only provides evidence on a very broad spatial and temporal scale (Redfern et al., 2006). As modern Antarctic blue whales have only been studied using PAM it is impossible to know the demographic structure or the exact numbers of the migratory population (figure 4); because the gender, proportion and age of calling whales is not known. Therefore studies which give quantitative results need to be employed in the future, such as tagging and mark-recapture, although there are likely to be logistical challenges with the latter method in Southern Ocean due to weather conditions.
Historic whaling records do show that a proportion of this population would travel to southwest Africa during the austral winter (Branch et al., 2007). Antarctic blues landed here had similar body characteristics to those caught off South Georgia, and the maximum catches in the region occurred during the winter when minimal catches were being made in Antarctica, matching the assumed migration cycle (Branch et al., 2007). However only two sightings have been reported since commercial whaling in the area ceased (Branch et al., 2007), suggesting this migratory subpopulation is likely extinct.
(b) Pygmy blue whales (B. m. brevicauda)
(i) Madagascan call type
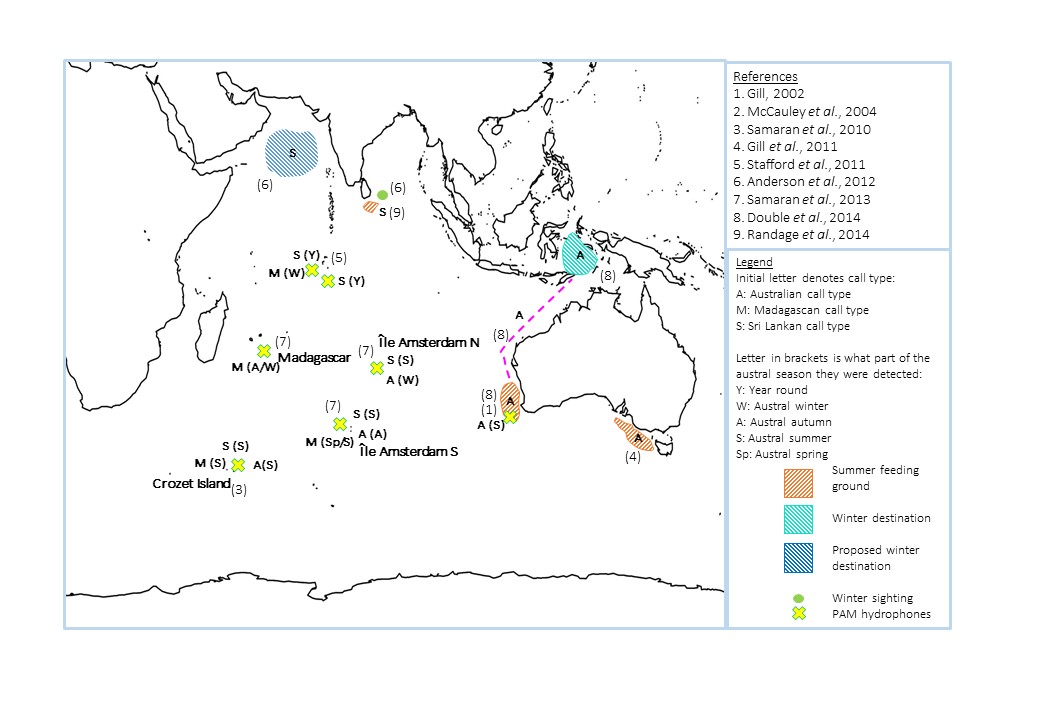
Results from PAM hydrophones show that these pygmy blue whales occupy the south-westerly portion of the Indian Ocean (figure 5). They are widely distributed during the austral summer; individuals have been recorded off eastern Madagascar at 27° S and at the Crozet Islands, a sympatric site for both pygmy and Antarctic blue whales, 2500 km further south (Samaran et al., 2010; Samaran et al., 2013). This suggests that at this time of the year the population is either diffusely spread out over a broad area, or that two subpopulations are present, each exploiting a different area of high productivity.
Figure 3. Summary of locations of Antarctic blue whales throughout the year. Numbers correspond to studies in the references box.
Figure 4. The variety of methods used to study blue whale populations since 1990. C. Pygmy = Chilean, A. Pygmy = Australian, S. Pygmy = Sri Lankan and M. Pygmy = Madagascan.
During the winter the frequency of calls increased at a PAM hydrophone off Île Amsterdam, compared to the stations of Madagascar and Crozet (Samaran et al., 2013). This could indicate that the diffuse summer population aggregates in this area, or that the two subpopulations converge. A review of pygmy blue whale distribution in the Indian Ocean basin found that aggregations correlate with regions of high primary productivity (a proxy for krill), even at their winter migratory destinations (Branch et al., 2007), contradicting the assumption that migration occurs primarily for breeding; instead, blue whales could be tracking krill resources year round.
As PAM is also the only method which has been used to study this population (figure 4), inferences on numbers and demographics moving between different regions cannot be made; other silent portions of the population could be moving elsewhere (Redfern et al., 2006). As pygmy blue whale populations cannot be physically distinguished, a new method, combining acoustics with tagging or mark-recapture could be developed, allowing certainty that a specific call-type was being monitored.
(ii) Sri Lankan call type
Due to the high number of calls recorded year-round in the northern Indian Ocean, Sri Lankan pygmy blue whales are considered to be mostly resident to this area (figure 5; Stafford et al., 2011); although a small number of calls have also been recorded in the Crozet sympatric region (figure 5; Samaran et al., 2013). Whale watching vessels off the southern coast of Sri Lanka sight these individuals year round, and small calves have been seen during the northeast monsoon, which creates highly productive waters in this region from November to April (Randage et al., 2014). This is unusual as it falls outside the assumed austral winter breeding season.
It has been suggested that during the other half of the year a partial westward migration to the Arabian Sea, to exploit the productivity of the southwest monsoon, takes place (Anderson et al., 2012). Although not all individuals migrate (Anderson et al., 2012), and travel to this destination needs to be confirmed with tagging or mark-recapture studies, it does strongly suggest that for this population at least, resource tracking is the main reason for migration. Consequently mating, and therefore calving, may take place opportunistically throughout the year, explaining why small calves are seen outside the austral winter.
Figure 5. Summary of the locations of the three Indian Ocean pygmy whale call types throughout the year. Numbers correspond to the studies in the references box .
(iii) Australian call type
The waters off southern and western Australia are important feeding grounds for this population of pygmy blue whales during the austral summer and autumn, with the highest frequency of calls, and therefore assumed numbers of individuals, recorded from February to May (figure 5; Gill, 2002; Stafford et al., 2011). The greater variety of methods used to study this population means that fine-scale information is available on one of their migratory routes and destinations (figure 4). A recent satellite telemetry study in which 11 whales were tagged over a two year period found that, at the cessation of upwelling off western Australia during the austral winter, they migrated north (Double et al., 2014). The whales initially stayed within 100 km of the coastline until reaching the Northwest Cape, when they moved a further 138 km offshore (Double et al., 2014).
Whales reached Indonesia by June, aggregating around the Banda and Molucca Seas. Although care needs to be taken when extrapolating these kinds of low power studies to population level (Redfern et al., 2006), the fact that all individuals followed a similar route does suggest the remainder of the population may also do so. It was assumed that the Indonesian end of the migration was a potential breeding ground (Double et al., 2014). However productivity is relatively high in these regions during the austral winter (Double et al., 2014), and the fact that no mating or calving has been witnessed does suggest that the tracking of productivity is also the primary reason for migration in this population.
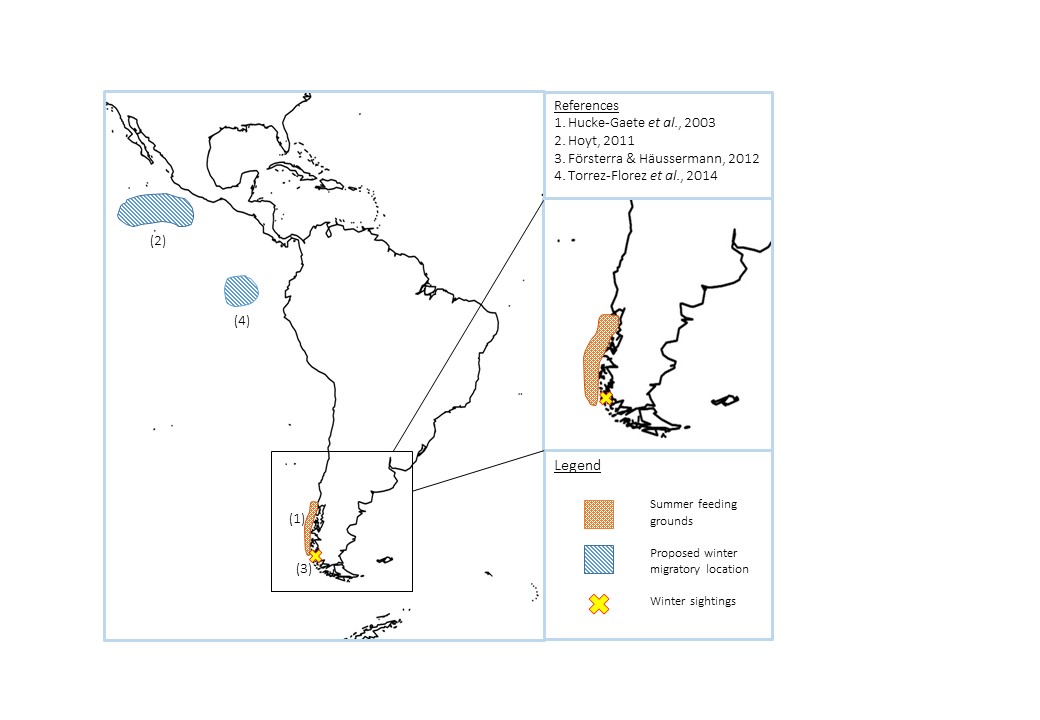
(iv) Chilean call type
Chilean pygmy blue whales aggregate within the fjords and islands of southern Chile during the austral summer (figure 6). Due to the high number of female-calf pairs sighted during opportunistic aerial surveys (11 out of 47 groups) this area has also been declared a key nursing ground for the population (Hucke-Gaeta et al., 2003). The geographic separation from other pygmy blue whales has led to some suggestions that they are a separate subspecies, but as of yet genetic studies have not found enough differentiation (Torres-Florez et al., 2014).
Figure 6. Summary of the locations of Chilean pygmy blue whales throughout the year. Numbers correspond to studies in the references box.
Very little is known about this population, the majority of studies have been based on opportunistic sightings (figure 4); therefore information on numbers and population structure remains unknown. However genetic studies have provided some preliminary evidence of the winter migratory destinations of these whales (Reilly & Thayer, 1990; Torres-Florez et al., 2014). Comparing mtDNA and nuclear DNA of Chilean pygmy blue whales with samples from other regions, no difference in haplotype frequency was found between the known population and samples from northern Chile, and the Eastern Tropical Pacific, which matches with sightings of blue whales off the Galapagos Islands during the austral winter (Branch et al., 2007; Torres-Florez et al., 2014). Due to the continual presence of blue whales around the Costa Rica Dome in the northern hemisphere, it has also been suggested that some Chilean pygmies may occupy this region during the austral winter, while the Californian blue whales are further north exploiting boreal summer productivity (Hoyt, 2011). However this is just speculation, and it is clear that before inferences on the motivation behind their migration can be made more in depth study needs to take place, especially as it appears a proportion of the population remain in southern Chile during the austral winter (Försterra & Häusserman, 2012).
(3) The Northern Hemisphere
(a) The North Atlantic Ocean
Whaling records for this area show a wide distribution in tropical and temperate waters during the boreal winter, becoming more concentrated and northerly during the summer (Reeves et al., 2004). Although this inferred distribution could be the result of effort bias associated with whalers primarily tracking northern right whale distribution (Reeves et al., 2004), there is more modern evidence to support this.
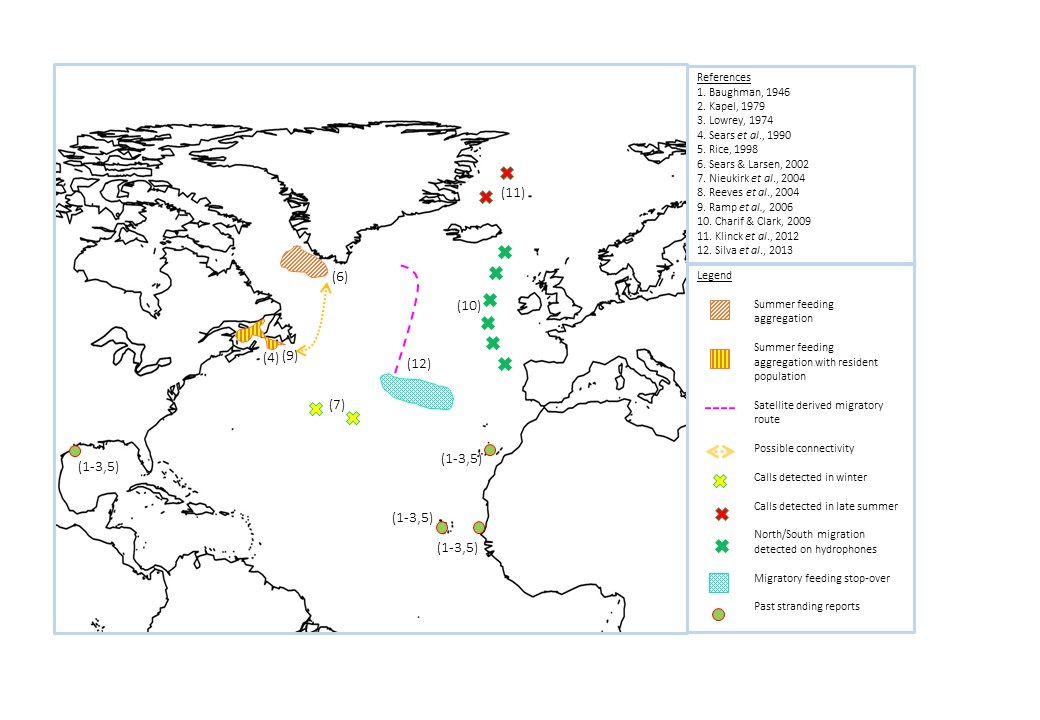
Their summer distribution is made up of interconnected feeding stocks in high latitude waters, with known aggregations in the Gulf of St Lawrence, west Greenland and west Iceland (figure 7). Blue whales show both fidelity and transfer between feeding areas across years; for example mark-recapture analysis showed that an individual utilised the Gulf in 1984 and 1985, followed by west Greenland in 1988 and 1989, returning to the Gulf from 1991-1994 (Sears & Larsen, 2002). Additionally a survival analysis has shown that many blue whales pass through the Gulf only occasionally, with up to 18 years between sightings (Ramp et al., 2006). Polar waters are also utilised during the boreal summer, although supposedly at much lower levels than the main aggregation areas (Klinck et al., 2012).
A proportion of the North Atlantic population do migrate to lower latitudes during the boreal winter, however a non-migratory, resident population is thought to be present within the Gulf of St Lawrence, as here blue whales are sighted almost year round from January to November (Sears et al., 1990). It is thought these individuals may only travel very short distances to Newfoundland during December (Sears et al., 1990). This resident population could occur due to the availability of resources year-round, however the selection processes determining which members of the population stay, and which are more nomadic remain a mystery.
Figure 7. Summary of the locations of North Atlantic blue whales throughout the year. Numbers correspond to studies in the references box.
For the proportion that do migrate south during the boreal summer, one potential migratory route is through the offshore waters of the British Isles; an array of 12 PAM hydrophones were able to track individuals moving south during the autumn and winter, and then north from April to June (figure 7; Charif & Clark, 2009). Around their low-latitude destinations, naval hydrophones have recorded blue whales around the mid-Atlantic ridge, most frequently during the winter (Nieukirk et al., 2004). This presence at lower latitudes is further supported by past strandings reported in Texas, Cape Verde, the Canary Islands and Senegal (Baughman, 1946; Kapel, 1979; Lowrey, 1979; Rice, 1998).
The migratory paradigm states that baleen whales fast while travelling and at their winter migratory destinations; however evidence from satellite telemetry suggests that North Atlantic blues feed at stop-overs enroute (Silva et al., 2013). Three individuals tagged off the Azores on their return migration exhibited area restricted search (ARS) behaviour, associated with foraging, in locations where their respective prey numbers were predicted to be higher (Silva et al., 2013). This shows that not only may the majority of blue whale populations migrate primarily to track primary productivity, but they also utilise mid-distance areas as stop overs where prey is present. It should be acknowledged that the sample size and duration of this study was very small, so before conclusions are made, further long-term studies tagging higher numbers of individuals should be carried out.
(b) The North Pacific Ocean
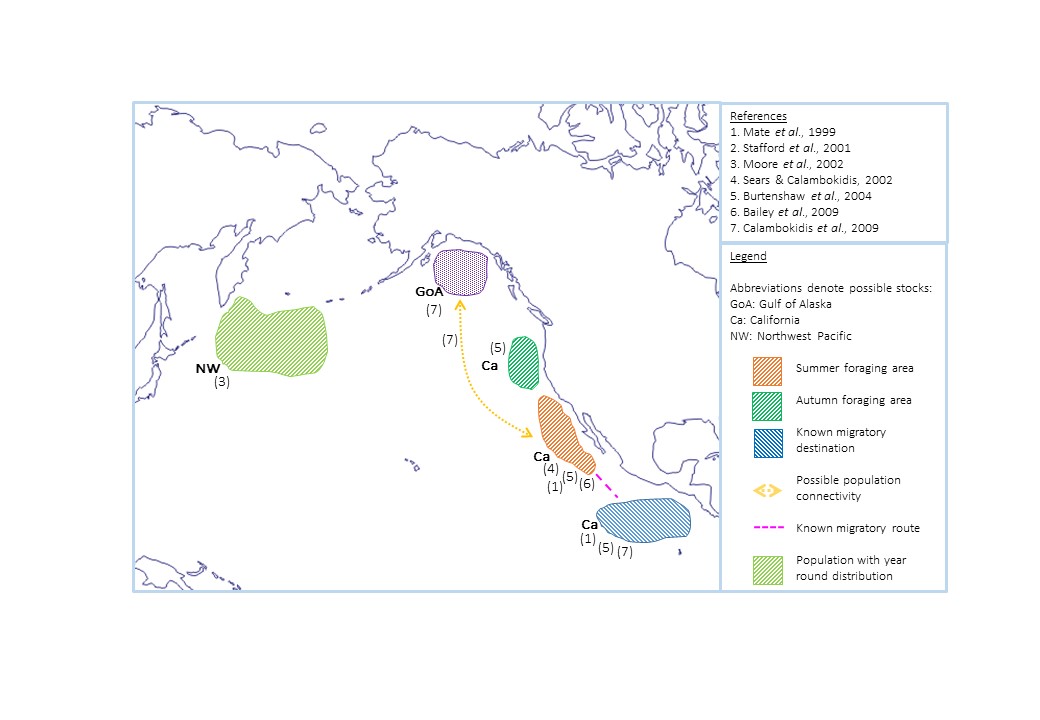
It is likely that at least two blue whale populations are present in the North Pacific Ocean, as a northeast and northwest vocal clan exists, each with very different yearly ranges and migratory patterns (Stafford et al., 2001). For the Northeast Pacific population, both satellite telemetry and PAM studies agree that during the late boreal summer, feeding aggregations occur off central and southern California, to exploit the euphausiid blooms facilitated by the California Current system (figure 8; Mate, 1999; Burtenshaw et al., 2004; Bailey et al., 2009; Block et al., 2011). A PAM study from 1994 to 2000 found that during the autumn some individuals travel north along the Oregon and Washington coasts to track the northward autumn plume of primary production, and therefore krill (Burtenshaw et al., 2004). It is thought that this movement might connect the Californian population with the small, whaling-depleted Gulf of Alaska (GoA) population; mark-recapture analysis of 15 individuals spotted in the GoA from 1997 to 2009 found that some were sighted in both areas (Calambokidis et al., 2009). However the very low numbers of blue whales in the GoA may mean that encounter rates will never be high enough to establish whether there is connectivity between these regions.
The Costa Rica Dome (CRD) is the main winter migratory destination of this population, as established from a satellite telemetry study of 159 whales (figure 8; Bailey et al., 2009). Since its discovery, it has been considered the most likely evidence of a blue whale calving and mating ground (Bailey et al., 2009); however, actual evidence is not conclusive. Calves have been sighted, but not in high abundances, and the only evidence of mating is reports of small, highly active groups of individuals; behaviour which is also seen off California (Hoyt, 2011). Area restricted search behaviour identified through tagging is much more likely a result of foraging activity. The CRD is a highly productive location, and feeding is occurring here, as red faeces (a result of eating red krill) has been sighted alongside blue whales (Brower, 2009; Hoyt, 2011). This gives conclusive evidence that they are feeding within this area, and therefore do not migrate exclusively to breed.
Figure 8. Summary of the locations of North Pacific blue whales throughout the year. Numbers correspond to studies in the references box.
In the Northwest Pacific call locations of the other population suggest that these individuals remain at high latitudes year round (figure 8; Watkins et al., 2000). Combining PAM with environmental data on a Geographic Information System (GIS) it was found that these blue whales closely associate with different topographical features of the ocean floor on an annual cycle (Moore et al., 2002). During the winter, calls were located primarily around the Emperor seamounts, moving to the end of the Kamchatka peninsula during spring. Over the summer they were widely spread across the Northwest Pacific, again aggregating near the Emperor seamounts during the autumn. These individuals appear to migrate within a smaller spatial area than their northeastern conspecifics, utilising diffuse productivity during the summer, and concentrating around localised productivity hotspots created by topographical features during less productive seasons (Moore et al., 2002). However it is important to consider that this is the only spatial use study (figure 4), and being PAM, it may not account for the whole population. Therefore further study with a variety of methods should be a priority in this area.
III. The potential impacts of climate change
As blue whale populations worldwide are reliant upon resources associated with high productivity year round, they could be particularly vulnerable to climate change effects. Being highly mobile and able to travel long distances between resources, it might be expected that they will be able to adapt to and track any changes in primary productivity associated with climate change (Robinson et al., 2009). It is true that natural climate oscillations take place over both short and long time scales in ocean basins, with blue whale populations showing some adaptive ability. The El Niño Southern Oscillation (ENSO) affects blue whale movement and habitat use along the US coastline. In El Niño years, PAM hydrophones detected a decrease in the calling amplitude in Californian waters, coupled with an increase in call amplitude further north around Vancouver (Burtenshaw et al., 2004). This was linked to a decrease in the abundance of krill around California associated with ENSO anomalies, whereas areas further north stayed productive (Burtenshaw et al., 2004). Current systems around California also show high inter-annual variability, and Northeast Pacific blue whales are able to track these changeable fine-scale thermal fronts which are associated with upwelling (Keiper et al., 2005; Doniol-Valcroze et al., 2007).
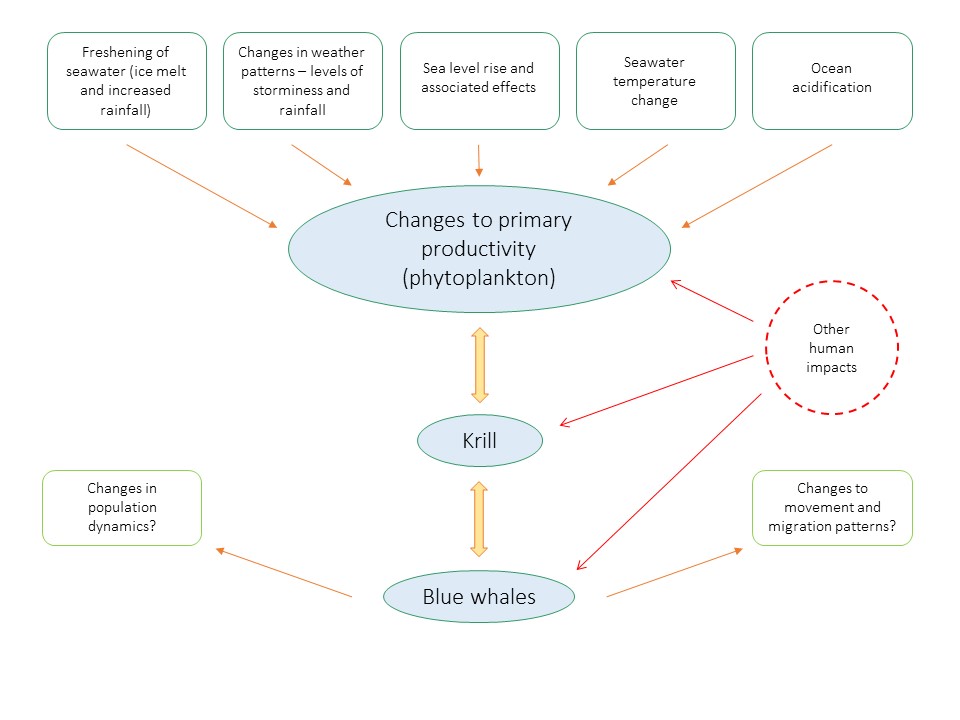
This demonstrates that some populations of blue whales may be able to adapt to changes in productivity, provided there are highly productive areas in other locations occurring at the same time. However climate change is not just expected to alter the spatial and temporal occurrence of resources, but their quantity as well (figure 9). As blue whales are highly selective predators, feeding almost entirely on krill, they may not be able to make adaptive prey switches like other species have, such as humpback whales (figure 9; Etnoyer et al., 2006; Fleming et al., 2015).
At high latitudes, climate change models predict that net primary productivity (NPP) may increase, due to longer growing seasons and better mixed-layer dynamics (Behrenfeld et al., 2006). Using model assumptions alone it would appear that krill would benefit from this, and therefore also blue whales. However in the Antarctic, krill survival is tightly coupled to sea ice cover (Montes-Hugo et al., 2009). In comparison to 1979, Antarctic sea ice now appears 54 days later and melts 10 days earlier (Montes-Hugo et al., 2009). The reduction in sea ice is means that krill distribution is contracting in a poleward direction (Montes-Hugo et al., 2009); the similar range shift seen in Antarctic blue whales could therefore be due to climate change, rather than overexploitation by whaling. With other impacts to krill alongside climate change, such as fishing in the Southern Ocean, Antarctic blue whales are likely to be the most vulnerable population at present (Wiedenmann et al., 2011).
Figure 9. Overall summary of how climate change may impact blue whale populations on a global scale. Adapted from Simmonds & Elliot (2009).
At low, tropical latitudes, Walker circulation, and inadvertently productivity, relies on western convection and eastern subsistence (Vecchi et al., 2006). Models have suggested that it has already decreased, and will continue to do so (Vecchi et al., 2006). This will slow equatorial currents, and cause a drop in the strength and depth of equatorial upwelling, in turn affecting productivity and prey availability (Vecchi et al., 2006). Populations which rely on tropical productivity year round, such as the monsoon-tracking Sri Lankan pygmy blue whales, may be particularly vulnerable. Changes in productivity in this region, caused by increased monsoon rainfall (a product of climate change), have already caused short-term changes in their distribution (de Vos et al., 2014). In 2011, higher levels of freshwater in the surface waters off Sri Lanka, caused by high rainfall, reduced the productivity of coastal waters, which altered the distribution of krill, and therefore the whales (de Vos et al., 2014). If climate associated productivity changes such as these begin to occur more regularly then the distribution (and migration routes) of blue whales could be altered on a much more long-term scale.
Climate change associated productivity shifts may mean that blue whales have to migrate larger distances searching for resources. For example the poleward shift in Antarctic blue whale summer distribution means that their winter migratory destinations are further away; which could have negative energetic and population effects, such as starvation and a lower calf birth rate. Altered distributions are already causing interbreeding between subspecies, and the creation of hybrids in previously non-sympatric areas, potentially leading to taxonomic changes in the future, as not all hybrids are sterile (Attard et al., 2012). Overall, any negative population effects associated with changes to migratory locations, prey abundance and distribution could cause localised extinctions, as populations are already at very low levels.
IV. How will climate change impact blue whale conservation?
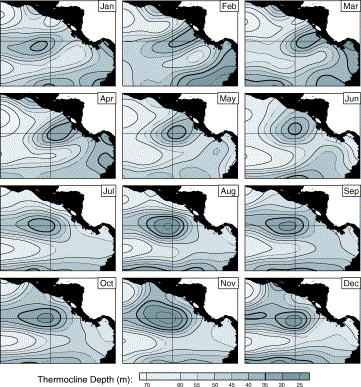
Due to the paucity of information on blue whale distribution and migration worldwide, current conservation efforts are based in areas of known aggregations of blue whales, which have the potential to change in the future. The Costa Rica Dome, which is thought to be utilised by blue whales from both hemispheres, is one such location which has been proposed as a high seas marine protected area, specifically for the species (Hoyt, 2011). This locality is particularly susceptible to climate change, as it is an upwelling plume with a thermocline that changes seasonally (figure 10; Fiedler, 2002). It cannot be defined by physical boundaries, and is only detectable through conductivity temperature depth (CTD) surveys (Hoyt, 2011). Although presently the seasonal changes in the position of the dome are known (figure 10), climate change is likely to cause the plume to become less productive or offset it from its current position. This may mean that the boundaries of the proposed protected area would not match with the high productivity areas that blue whales are exploiting. Alternatively if the upwelling were to reduce in strength the whales could begin to favour another site entirely, meaning that protection would be better allocated elsewhere.
Figure 10. Monthly mean changes in the position of the Costa Rica Dome (thick black line). This highlights the difficulty in defining a protected area, even without the influence of climate change. Figure from Fiedler (2002).
The management of specific threats, such as ship strikes, could also be impacted if the migratory pathways of blue whales were to alter as a result of climate change. Off the coast of Sri Lanka blue whales occur within an area of very high shipping traffic at known times of the year (Randage et al., 2014). Mandatory ship reporting systems (MSRS) and speed limits within these predictable areas has been suggested as a method of reducing strikes (Randage et al., 2014). This has already been applied with some success on other species, such as northern right whales, Eubalaena glacialis, in the North Atlantic (Ward-Geiger et al., 2005). If a change in the distribution of these blue whales was to move them outside the MSRS area, they could be more vulnerable to ship related injuries and fatalities.
Any changes in distribution and movement pathways which take blue whale populations outside of protected areas or into dangerous regions could lead to increased anthropogenic-associated mortality. Coupled with the negative population effects likely to be caused by climate change, this could further accelerate a possible population decline. As such it is important to continue and establish new blue whale monitoring programmes so that marine protected areas and conservation measures can be implemented or adapted in the right locations.
V. Conclusions
It is clear that blue whales do not fit the current paradigm of migration applied to many baleen whale species. Individuals feed throughout the year, and either over-winter in the same region, or migrate to a variety of productive lower latitude locations singly or in small groups. Breeding areas have been proposed but not discovered; so it is likely that mating, and therefore calving, occurs opportunistically or is of secondary importance to winter resource tracking. This highlights that the migratory cycle of a very well-known species, should not be expanded into a paradigm to cover lesser known species, even if they are closely related genetically.
The movement patterns and distribution of blue whales in the Indian, North Atlantic and North Pacific Oceans have been relatively well studied in comparison to other areas. However within these regions information gaps are still present. Research needs to be prioritised towards finding the destinations of the southward migration of blue whales in the North Atlantic, and discovering whether Sri Lankan pygmy blue whales utilise the productivity of the southwest monsoon in the Arabian Sea, as suggested. Establishing the connectivity between the GoA blue whales and the Californian population should also be a priority, but low numbers in the GoA make this challenging.
In the South Pacific the migratory destinations of Chilean pygmy blue whales (including the use of the Costa Rica Dome) need to be established, as evidence of these areas currently comes from speculation and a single genetic study. In the future the Antarctic subspecies needs to be studied with a greater variety of methods; using only PAM has given no information on migratory numbers or finer-scale movement corridors. Information paucity in many populations would be improved through satellite telemetry studies, although sample numbers would need to be large to improve reliability when movement patterns are extrapolated to population level. However, overall research effort should be concentrated on forming a blue whale monitoring program in the South Atlantic, as no research has been conducted on their distribution and movements in this basin.
As climate change is predicted to alter the locations and strength of primary productivity, it is likely that the locations and abundances of krill swarms, and therefore blue whales, will also change. Populations which inhabit areas with high annual variability, such as the northeast Pacific, which is subject to climate oscillations and the variable California Current System, may be able to adapt to these changes. However in other areas, such as the Southern Ocean, where krill are declining in numbers due to receding sea ice, it may have detrimental population effects on the Antarctic blue whale inhabiting this region. Overall these changes are likely to alter the locations of blue whale aggregations and their migratory pathways.
Resultantly there could be large knock on impacts for conservation, as blue whales may move outside protected regions, and therefore be at greater risk from other anthropogenic impacts such as ship strikes. Increased frequency of anthropogenic mortality associated with spending more time outside protective areas could be additive to the negative impacts of climate change. Additionally if blue whales move outside monitoring areas these changes may not be detected. Movement changes could be identified in the future, and protected area boundaries changed or created accordingly; however this will only be possible if a baseline is created from which deviation can be detected. Therefore additional and continued research effort in all ocean areas is needed, covering blue whale movement and climate associated krill and productivity changes.
VI. Glossary
Area-restricted search – a track pattern which is part of a state-space model approach. Rather than lines being straight with a fast swimming speed, tracks are highly sinuous within a restricted area, and swimming speeds are lower
Availability bias – the non-counting of individuals which cannot be seen below the surface during surveys
Net primary productivity – the total energy produced by phytoplankton photosynthesis minus the energy they use during respiration
Paradigm – a scientific theory developed from many studies and research papers
Perception bias – the non-counting of individuals which are missed by observers on surveys
VII. References
Anderson, R.C., Branch, T.A., Alagiyawadu, A., Baldwin, R & Marsac, F. (2012). Seasonal distribution, movements and taxonomic status of blue whales (Balaenoptera musculus) in the northern Indian Ocean. Journal of Cetacean Research and Management, 12, 203-218.
Attard, C.R.M., Beheregaray, L.B., Jenner, K.C.S, Gill, P.C., Jenner, M.N., Morrice, M.G., Robertson, K.M. & Möller, L.M. (2012). Hybridisation of Southern Hemisphere blue whale subspecies and a sympatric area off Antarctica: impacts of whaling or climate change? Molecular Ecology, 21, 5715-5727.
Bailey, H., Mate, B.R., Palacios, D.M., Irvine, L., Bograd, S.J. & Costa, D.P. (2009). Behavioural estimation of blue whale movements in the Northeast Pacific from state-space model analysis of satellite tracks. Endangered Species Research, 10, 93-106.
Baughman, J.L. (1946). On the occurrence of a rorqual whale on the Texas coast. Journal of Mammology, 27, 392-393.
Behrenfeld, M.J., O’Malley, R.T., Siegel, D.A., McClain, C.R., Sarmiento, J.L., Feldman, G.C., Milligan, A.J., Falkowski, P.G., Letelier, R.M. & Boss, E.S. (2006). Climate-driven trends in contemporary ocean productivity. Nature, 444, 752-755.
Berman-Kowalewski, M., Gulland, F.M.D., Wilkin, S., Calambokidis, J., Mate, B., Cordaro, J., Rostein D., St.Leger, J., Collins, P., Fahy, K. & Dover, S. (2010). Association between blue whale (Balaenoptera musculus) mortality and ship strikes along the Californian coast. Aquatic Mammals, 36, 59-66.
Block, B.A., Jonsen, I.D., Jorgensen, S.J., Winship, A.J., Shaffer, S.A., Bograd, S.J., Hazen, E.L., Foley, D.G., Breed, G.A., Harrison, A.L., Ganong, J.E., Swithenbank, A., Castleton, M., Dewar, H., Mat, B.R., Shillinger, G.L., Schaefer, K.M., Benson, S.R., Weise, M.J., Henry, R.W. & Costa, D.P. (2011). Tracking apex marine predator movements in a dynamic ocean. Nature, 475, 86-90.
Bose, N. & Lien, J. (1989). Propulsion of a fin whale (Balaenoptera physalus): why the fin whale is a fast swimmer. Proceedings of the Royal Society of London B: Biological Sciences, 237, 175-200.
Branch, T.A., Stafford, K.M., Palacios, D.M., Allison, C., Bannister, J.L., Burton, C.L.K., Cabrera, E., Carlson, C.A., Galletti-Vernazzani, B., Gill, P.C., Hucke-Gaete, R., Jenner, K.C.S, Jenner, M.N.M, Matsuoka, K., Mikhalev, Y.A., Miyashita, T., Morrice, M.G., Nishiwaki, S., Sturrock, V.J., Tormosov, D., Anderson, R.C., Baker, A.N., Best, P.B., Borsa, P., Brownwell Jr, R.L., Childerhouse, S., Findlay, K.P., Gerrodette, T., Ilangakoon, A.D., Joergensen, M., Kahn, B., Ljungblan, D.K., Maughan, B., McCauley, R.D., McKay, S., Norris, T.F., Oman Whale and Dolphin Research Group, Rankin, S., Samaran, F., Thiele, D., Van Waerebeek, K. & Warneke, R.M. (2007). Past and present distribution, densities and movements of blue whales Balaenoptera musculus, in the Southern Hemisphere and northern Indian Ocean. Mammal Review, 37, 116-175.
Brower, K. (2009). ‘Still blue’. National Geographic, 215, 134-153.
Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L., Borchers, D.L., & Thomas, L. (2001). Introduction to distance sampling estimating abundance on biological populations. Oxford University Press, Oxford.
Burtenshaw, J.C., Oleson, E.M., Hildebrand, J.A., McDonald, M.A., Andrew, R.K., Howe, B.M. & Mercer, J.A. (2004). Acoustic and satellite remote sensing of blue whale seasonality and habitat in the Northeast Pacific. Deep-Sea Research II, 51, 967-986.
Calambokidis, J., Barlow, J., Ford, J.K.B., Chandler, T.E. & Douglas, A.B. (2009). Insights into the population structure of blue whales in the Eastern North Pacific from recent sightings and photographic identification. Marine Mammal Science, 25, 816-832.
Charif, R.A. & Clark, C.W. (2009). Acoustic monitoring of large whales in deep waters north and west of the British Isles: 1996-2005. Technical Report 08-07, Cornell Lab of Ornithology: Bioacoustics Research Program.
Clapham, P. (2001). Why do baleen whales migrate? Marine Mammal Science, 17, 432-436.
Conway, C. (2005). Analysis of blue whale (Balaenoptera musculus) population structure worldwide using the variation contained within introns of conserved nuclear genes. Ph.D. thesis, University of California, Davis, CA.
Corkeron, P.J. & Connor, R.C. (1999). Why do baleen whales migrate? Marine Mammal Science, 15, 1228-1245.
de Vos, A., Pattiaratchi, C.B. & Harcourt, R.G. (2014). Inter-annual variability in blue whale distribution off southern Sri Lanka between 2011 and 2012. Journal of Marine Science and Engineering, 2, 534-550.
Deméré, T.A., McGowen, M.R., Berta, A. & Gatesy, J. (2008). Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Systematic Biology, 57, 15-37.
di Sciara, G.N. & Gordon, J. (1997). Bioacoustics: a tool for the conservation of cetaceans in the Mediterranean Sea. Marine and Freshwater Behaviour and Physiology, 30, 125-146.
Dingle, H. (2014). Migration: the biology of life on the move. Oxford University Press, Oxford.
Doniol-Valcroze, T., Berteaux, D., Larouche, P. & Sears, R. (2007). Influence of thermal fronts on habitat selection by four rorqual whale species in the Gulf of St Lawrence. Marine Ecology Progress Series, 335, 207-216.
Double, M.C., Andrews-Goff, V., Jenner, K.C.S., Jenner, M.N.M., Laverick, S.M., Branch, T.A. & Gales, N.J. (2014). Migratory movements of pygmy blue whales (Balaenoptera musculus brevicauda) between Australia and Indonesia as revealed by satellite telemetry. PloS one, 9, e93578.
Etnoyer, P., Canny, D., Mate, B.R., Morgan, L.E., Ortega-Ortiz, J.G. & Nichols, W.J. (2006). Sea-surface temperature gradients across blue whale and sea turtle foraging trajectories off the Baja California Peninsula, Mexico. Deep-Sea Research II, 53, 340-358.
Fiedler, P.C., Reilly, S.B., Hewitt, R.P., Demer, D., Philbrick, V.A., Smith, S., Armstrong, W., Croll, D.A., Tershy, B.R. & Mate, B.R. (1998). Blue whale habitat and prey in the California Channel Islands. Deep-Sea Research II, 45, 1781-1801.
Fiedler, P.C. (2002). The annual cycle and biological effects of the Costa Rica Dome. Deep-Sea Research I, 49, 321-328.
Fleming, A.H., Clark, C.T., Calambokidis, J. & Barlow, J. (2015). Humpback whale diets respond to variance in ocean climate conditions in the California Current. Global Change Biology, 22, 1214-1224.
Fösterra, G. & Häussermann, V. (2012). Report on blue whales sightings (Balaenoptera musculus Linneaus, 1758) in a narrow fjord during autumn-winter in southern Chile. Spixana, 35, 237-245.
Gregr, E.J. (1992). An analysis of historic (1908-1967) whaling records from British Columbia, Canada. Masters Thesis. The University of British Columbia, Canada.
Gill, P.C. (2002). A blue whale (Balaenoptera musculus) feeding ground in a southern Australian coastal upwelling zone. Journal of Cetacean Research and Management, 4, 179-184.
Gill, P.C., Morrice, M.G., Page, B., Pirzl, R., Levings, A.H. & Coyne, M. (2011). Blue whale habitat selection and within-season distribution in a regional upwelling system off southern Australia. Marine Ecology Progress Series, 421, 243-263.
Hoyt, E. (2011). Marine Protected Areas for Whales, Dolphins and Porpoises: A world handbook for cetacean habitat conservation and planning. Routledge Press, London.
Hucke-Gaete, R., Osman, L.P., Moreno, C.A., Findlay, K.P. & Ljungblad, D.K. (2003). Discovery of a blue whale feeding and nursing ground in southern Chile. Biology Letters, 271, 170-173.
Ichihara, T. (1966). The pygmy blue whale, Balaenoptera musculus brevicauda, a new subspecies from the Antarctic. In Whales, dolphins and porpoises (eds K.S. Norris), pp. 79-111. University of California Press.
Kapel, F.O. (1979). Exploitation of large whales in West Greenland in the twentieth century. International Whaling Commission Reports SC/30/DOC, 23, 197-214.
Keiper, C.A., Ainley, D.G., Allen, S.G. & Harvey, J.T. (2005). Marine mammal occurrence and ocean climate off central California, 1986 to 1994 and 1997 to 1999. Marine Ecology Progress Series, 289, 285-306.
Klinck, H., Nieukirk, S.L., Mellinger, D.K., Klinck, K., Matsumoto, H. & Dziak, R.P. (2012). Seasonal presence of cetaceans and anthropogenic noise levels in polar waters of the North Atlantic. Journal of the Acoustical Society of America, 132, 176-181.
Lascelles, B., di Sciara, G.N., Agardy, T., Cuttelod, A., Eckert, S., Glowka, L., Hoyt, E., Llewellyn, F., Louzao, M., Ridoux, V. & Tetley, M.J. (2014). Migratory marine species: their status, threats and conservation management needs. Aquatic Conservation: Marine and Freshwater Ecosystems, 24, 111-127.
Lowrey, G.H. (1974). Mammals of Louisiana and its adjacent waters. Louisiana State Press, Louisiana.
Mackintosh, N.A., Wheeler, J.F.G. & Clowes, A.J. (1929). Southern blue and fin whales. University Press.
Marsh, H. & Sinclair, D.F. (1989). Correcting for visibility bias in strip transect aerial surveys of aquatic fauna. Journal of Wildlife Management, 53, 1017-1024.
Mate, B.R., Lagerquist, B.A. & Calambokidis, J. (1999). Movements of North Pacific blue whales during the feeding season off southern California and their southern fall migration. Marine Mammal Science, 15, 1246-1257.
McCauley, R., Bannister, J., Burton, C., Jenner, C., Rennie, S., & Kent, C.S. (2004). Western Australian exercise area blue whale project. Final Summary Report. Milestone, 6.
Metcalfe, C., Koenig, B., Metcalfe, T., Paterson, G. & Sears, R. (2004). Intra- and inter-species differences in persistent organic contaminants in the blubber of the blue whales and humpback whales from the Gulf of St Lawrence, Canada. Marine Environmental Research, 57, 245-260.
Montes-Hugo, M., Doney, S.C., Ducklow, H.W., Fraser, W., Martinson, D., Stammerjohn, S.E. & Schofield, O. (2009). Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science, 323, 1470-1473.
Moore, S.E., Watkins, W.A., Daher, M.A., Davies, J.R. & Dahlheim, M.E. (2002). Blue whale habitat associations in the Northwest Pacific: analysis of remotely-sensed data using a Geographic Information System. Oceanography, 15, 20-25.
Moore, S.E., Grebmeier, J.M. & Davies, J.R. (2003). Gray whale distribution relative to forage habitat in the northern Bering Sea: current conditions and retrospective summary. Canadian Journal of Zoology, 81, 734-742.
Nieukirk, S.L., Stafford, K.M., Mellinger, D.K., Dziak, R.P. & Fox, C.G. (2004). Low-frequency whale and seismic airgun sounds recorded in the mid-Atlantic Ocean. Journal of the Acoustical Society of America, 115, 1832-1843.
Palacios, D.M. (1999). Blue whale (Balaenoptera musculus) occurrence off the Galápagos Islands, 1978-1995. Journal of Cetacean Research and Management, 1, 41-51.
Pavia, M.P. & Grangeiro, B.F. (1970). Investigations on the whaling seasons 1964-1967, off northeastern coast of Brazil. Arquicos de Ciências do Mar, 10, 111-126.
Pike, D.G., Vikingsson, G.A., Gunnlaugsson, T. & Øien, N. (2009). A note on the distribution and abundance of blue whales (Balaenoptera musculus) in the central and northeast North Atlantic. North Atlantic Marine Mammal Commission Scientific Publications, 7, 19-29.
Pitman, R.L., Durban, J.W., Greenfelder, M., Guinet, C., Jorgensen, M., Olson, P.A., Plana, J., Tixier, P. & Towers, J.R. (2011). Observations of a distinctive morphotype of killer whale (Orcinus orca), type D, from subantarctic waters. Polar Biology, 34, 303-306.
Ramp, C., Bérubé, M., Hagen, W. & Sears, R. (2006). Survival of adult blue whales Balaenoptera musculus in the Gulf of St. Lawrence, Canada. Marine Ecology Progress Series, 319, 287-295.
Ramp, C., Delarue, J., Palsbøll, P.J., Sears, R. & Hammond, P.S. (2015). Adapting to a warmer ocean – seasonal shift of baleen whale movements over three decades. PloS one, 10, e0121374.
Randage, S.M., Alling, A., Currier, K & Heywood, E. (2014). Review of the Sri Lanka blue whale (Balaenoptera musculus) with observations on its distribution in the shipping lane. Journal of Cetacean Research and Management, 14, 43-49.
Redfern, J.V., Ferguson, M.C., Becker, E.A., Hyrenbach, K.D., Good, C., Barlow, J., Kaschner, K., Baumgartner, M.F., Forney, K.A., Balance, L.T., Fauchald, P., Halpin, P., Hamazaki, T., Pershing, A.J., Qian, S.S., Read, A., Reilly, S.B., Torres, L. & Werner, F. (2006). Techniques for cetacean-habitat modelling. Marine Ecology Progress Series, 310, 271-295.
Reeves, R.R., Smith, T.D., Josephson, E.A., Clapham, P.J. & Woolmer, G. (2004). Historical observations of humpback and blue whales in the North Atlantic Ocean: clues to migratory routes and possibly additional feeding grounds. Marine Mammal Science, 20, 774-786.
Reilly, S.B. & Thayer, V.G. (1990). Blue whale (Balaenoptera musculus) distribution in the Eastern Tropical Pacific. Marine Mammal Science, 6, 265-277.
Rice, D.W. (1998). Marine mammals of the world: systematics and distribution. Society for Marine Mammology, Special Publication 4.
Robbins, J., Rosa, L.D., Allen, J.M., Mattila, D.K., Secchi, E.R., Friedlaender, A.S., Stevick, P.T., Nowacek, D.P. & Steel, D. (2011). Return movement of a humpback whale between the Antarctic Peninsula and American Samoa: a seasonal migration record. Endangered Species Research, 13, 117-121.
Robinson, R.A., Crick, H.Q.P., Learmonth, J.A., Maclean, I.M.D., Thomas, C.D., Bairlein, F., Forchhammer, M.C., Francis, C.M., Gill, J.A., Godley, B.J., Harwood, J., Hays, G.C., Huntley, B., Hutson, A.M., Pierce, G.J., Rehfisch, M.M., Sims, D.W., Santos, M.B., Sparks, T.H., Stroud, D.A. & Visser, M.E. (2009). Travelling through a warming world: climate change and migratory species. Endangered Species Research, 7, 87-99.
Roman, J. & Palumbi, S.R. (2003). Whales before whaling in the North Atlantic. Science, 301, 508-510.
Samaran, F., Adam, O. & Guinet, C. (2010). Discovery of a mid-latitude sympatric area for two Southern Hemisphere blue whale subspecies. Endangered Species Research, 12, 157-165.
Samaran, F., Stafford, K.M., Branch, T.A., Gedamke, J., Royer, J.Y., Dziak, R.P. & Guinet, C. (2013). Seasonal and geographic variation of southern blue whale subspecies in the Indian Ocean. PloS one, 8, e71561.
Sears, R. & Calambokidis, J. (2002). Update COSEWIC status report on the blue whale Balaenoptera musculus in Canada. In COSEWIC assessment and update status report on the blue whale Balaenoptera musculus in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, pp 1-32.
Sears, R. & Larsen, F. (2002). Long range movements of a blue whale (Balaenoptera musculus) between the Gulf of St. Lawrence and west Greenland. Marine Mammal Science, 18, 281-285.
Sears, R. & Perrin, W.F. (2009). Blue whale (Balaenoptera musculus). In Encyclopedia of Marine Mammals (eds W.F. Perrin, B. Würsig, J.G.M. Thewissen). Academic Press, San Diego, CA, pp 120-124.
Sears, R., Williamson, J.R., Wenzel, F.W., Bérubé, M., Gendron, D. & Jones, P.W. (1990). Photographic identification of the blue whale (Balaenoptera musculus) in the Gulf of the St Lawrence, Canada. Reports of the International Whaling Commision, 12, 335-342.
Silva, M., Prieto, R., Jonsen, I., Baumgartner, M.F. & Santos, R.S. (2013). North Atlantic blue and fin whales suspend their spring migration to forage in middle latitudes: building up energy reserves for the journey? PloS one, 8, e76507.
Simmonds, M.P. & Elliot, W.J. (2009). Climate change and cetaceans: concerns and recent developments. Journal of the Marine Biological Association of the United Kingdom, 89, 203-210.
Simmonds, M.P. & Isaac, S.J. (2007). The impacts of climate change on marine mammals: early signs of significant problems. Oryx, 41, 19-26.
Širović, A., Hildebrand, J.A., Wiggins, S.M., McDonald, M.A., Moore, S.E. & Thiele, D. (2004). Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep-Sea Research II, 51, 2327-2344.
Širović, A., Hildebrand, J.A., Wiggins, S.M. & Thiele, D. (2009). Blue and fine whale acoustic presence around Antarctica during 2003 and 2004. Marine Mammal Science, 25, 125-136.
Stafford, K.M., Nieukirk, S.L. & Fox, C.G. (2001). Geographic and seasonal variation of blue whale calls in the North Pacific. Journal of Cetacean Research and Management, 3, 65-76.
Stafford, K.M., Bohnenstiehl, D.R., Tolstoy, M., Chapp, E., Mellinger, D.K. & Moore, S.E. (2004). Antarctic-type blue whale calls recorded at low latitudes in the Indian and eastern Pacific Oceans. Deep-Sea Research I, 51, 1337-1346.
Stafford, K.M., Chapp, E., Bohnenstiel, D.R. & Tolstoy, M. (2011). Seasonal detection of three types of “pygmy” blue whale calls in the Indian Ocean. Marine Mammal Science, 27, 828-840.
Thomisch, K., Boebel, O., Clark, C.W., Hagen, W., Spiesecke, S., Zitterbart, D.P. & Opzeeland, I.V. (2016). Spatio-temporal patterns in acoustic presence and distribution of Antarctic blue whales Balaenoptera musculus intermedia in the Weddell Sea. Endangered Species Research, 30, 239-253.
Torres-Florez, J.P., Hucke-Gaete, R., Leduc, R., Lang, A., Taylor, B., Pimper, L.E., Bedriñana-Romano, L., Rosenbaum, H.C. & Figueroa, C.C. (2014). Blue whale population structure along the eastern South Pacific Ocean: evidence for more than one population. Molecular Ecology, 23, 5998-6010.
Vecchi, G.A., Soden, B.J., Wittenberg, A.T., Held, I.M., Leetmaa, A. & Harrison, M.J. (2006). Weakening of tropical Pacific atmosphere circulation due to anthropogenic forcing. Nature, 441, 73-76.
Watkins, W.A., George, J.E., Daher, M.A., Mullin, K., Martin, D.L., Haga, S.H. & DiMarzio, N.A. (2000). Seasonality and distribution of whale calls in the North Pacific. Oceanography, 13, 62-67.
Wiedenmann, J., Cresswell, K.A., Goldbogen, J., Potvin, J. & Mangel, M. (2011). Exploring the effects of reduction in krill biomass in the Southern Ocean on blue whales using a state-dependent foraging model. Ecological Modelling, 222, 3366-3379.
Wilcove, D.S. & Wilkewski, M. (2008). Going, going, gone: is animal migration disappearing. PloS one, 6, e188.